Reactive Arthritis and the Chronic Lyme Disease Debate: Dissecting the Research 11/20/2012
Posted by thetickthatbitme in Diagnosis, Peer-Reviewed, Tick-Lit, Treatment.Tags: antigens, Arthritis, Borrelia, Chronic Lyme disease, health, Infectious Diseases Society of America, joints, Journal of Clinical Investigation, Lyme, Lyme Disease, medicine, mouse, research, spirochete, steroids, Yale
7 comments
As you may be aware, there is a great deal of controversy about persistence of Lyme disease symptoms after treatment—even when treatment involves long-term antibiotics. There are many theories about this, and several of them may be true, depending on the patient. The Centers for Disease Control (CDC) and the Infectious Diseases Society of America (IDSA) say that once you’ve been treated with a month of antibiotics, you no longer have a Borrelia burgdorferi infection. If you’re still experiencing symptoms, they call it Post-Lyme or antibiotic-refractory arthritis. At the other end of the spectrum, some Lyme-literate medical doctors (LLMDs) believe that Lyme is a chronic disease, and once you have it, you’ll have it for life. Long-term (read: indefinite) antibiotic treatment, they maintain, is necessary to keep the organisms from multiplying, but you’ll never fully be rid of them.
My views fall somewhere between these two extremes. (Disclaimer: I am not a medical professional.) On the one hand, I don’t think that four weeks of antibiotics, whether oral or intravenous, is really enough to kill off a Borrelia infection in a patient who’s been infected for years. (And this view is supported by Embers et al’s study of Rhesus macaques.) On the other hand, I don’t think that antibiotics-for-life is the answer either. There are just too many people who have been on antibiotics for years who don’t seem to be getting better. Plus there’s the fact that antibiotics can cause a lot of damage if you take them long-term, which significantly lowers the quality of life for people on these treatment regimens.
So when patients have been treated and they’re still experiencing symptoms, I see several possible explanations:
1) The antibiotics didn’t kill off all the bacteria, and they are still hanging around somewhere—perhaps hidden in joints, cartilage, or the brain. This, as you can imagine, is very difficult to prove, especially in living human beings.
2) The antibiotics killed off all the bacteria, but the patient was bitten by another tick and re-infected. This is highly possible if the patient’s environment, lifestyle, and preventive measures have not changed. It’s also difficult to detect when patients are only being follow-up tested with Western Blots, and not something like the C6 antibody assay, which gives you a titer so you can see if your antibodies to the bacteria suddenly increase. (A study related to reinfection was just published on Wednesday in the New England Journal of Medicine. You can read about it in the NY Times here, or read the study abstract. I’ll be doing a run-down of that study next week.)
3) The antibiotics killed off the bacteria, but the body is still making an immune response, possibly attacking its own cells, causing inflammation and continuing symptoms.
When new research comes out, I like to pay attention to see which of these explanations is supported and why. In this post, I’ll take you through a study called “Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy” that was conducted by some researchers at Yale and published in the Journal of Clinical Investigation back in June. (If you want to read along, you can access the full article here.)
Some Background:
The underlying question in this study is: what causes Lyme-associated arthritis in patients who have been treated with antibiotics? Is it that infectious spirochetes are still hiding somewhere in the body, or is it possible that antibiotics “kill” all the bacteria (read: disassemble them so they can’t multiply) but leave their building blocks (referred to as antigens because the body still detects them) behind, causing inflammation.
Let me explain how killing Borrelia in the human body works. The things you need to kill Borrelia are antibodies, first and foremost. If you don’t generate an IgM response, this infection can be fatal in the first 3 to 7 days. We know that IgM has a direct bactericidal effect. In other words, this antibody can kill Borrelia on its own in the absence of complement. IgG, by comparison, is very inefficient at killing Borrelia, but we make that, too. We also need phagocytes to kill Borrelia, and in order to generate antibodies, we need B cells that work. Another thing we need is toll-like receptors (TLRs). These are important for helping antibodies bind to pathogens or parts of pathogens. If we’re deficient in TLR2, or a certain molecule in TLR called myeloid differentiation antigen 88 (abbreviated MyD88), we can have an overwhelming infection.
What they did:
In this study, the researchers used a type of mouse in which this MyD88 protein has been knocked out—i.e. the mouse is totally deficient in MyD88. For that reason, they call it a Myd88-/- mouse. (No, that thing at the end is not an emoticon.) The problem with these mice is that they die quickly of opportunistic infection (specifically, Pneumocystis carinii), so in the lab, they have to give them an antibiotic called Sulfatrim or Septra, which is actually a combination of two antibiotics—sulfamethoxazole and trimethoprim. I’ll come back to why this is important a little later.
Now, an interesting thing is that this same group of researchers did a study back in 2002 using C3H mice, who don’t have the Myd88 protein knocked out—so essentially “normal” mice, in terms of their immune systems—and they showed that live Borrelia persist for 3 months after optimal treatment. These Borrelia that remained were sort of mutant Borrelia because they were missing a couple of proteins that they might need to infect other animals. The researchers knew this because the clean ticks that bit them got B. burgdorferi, but when they had those ticks bite healthy mice, those mice didn’t get infected.
Anyway, for some reason, these researchers didn’t want to use the C3H mice for this study, and they decided to use the Myd88-/- mice, who develop overwhelming infections. They also used a WT strain of mouse (which is not missing Myd88) for the sake of comparison. They infected both types of mice, and then they treated some of them with Doxycycline (through their water supply) and others with Ceftriaxone (via subcutaneous injection). Interestingly, they showed that after one day of Ceftriaxone therapy, they could kill all of the Borrelia. They also used an interesting microscopic technique which allows one to look real-time at tissue and watch an organism to see what it does. By cutting down to a mouse’s tendon, they can see what’s going on down there.
Their Findings:
“B. burgdorferi DNA can be detected in B6 Myd88-/-, but not WT, mice after treatment with Doxycycline” (p. 2).
Translation: After treating the mice with Doxycycline, the researchers couldn’t find any B. burgdorferi DNA in the normal immune system mice, but they could find the DNA in the immune-compromised mice. One of those mice had a positive culture for B. burgdorferi, and ticks that fed on that mouse also tested positive for B. burgdorferi. HOWEVER, when they took samples from the knee joints of the mice, ALL of the mice tested positive for the ospA plasmid (B. burgdorferi DNA). In addition, ear-skin samples from half the immune-compromised mice and one of the normal mice tested positive for B. burgdorferi DNA (p. 2).
“Real-time imaging of B. burgdorferi in Myd88-/- mice reveals rapid spirochete elimination after antibiotic therapy” (p. 2).
Translation: The researchers used intravital 2-photon microscopy to observe the behavior of the B. burgdorferi spirochetes in the infected mice. Specifically, they looked at the dermis (skin) and the calcaneal (achilles) tendons. They say that 24 hours after beginning treatment with Ceftriaxone, the number of spirochetes had “diminished dramatically” in both the skin and tendons. The spirochetes left behind in the skin appeared to be moving, but the ones in the tendons did not. The following day, they were not able to see any spirochetes using this imaging technique, suggesting that they had all been destroyed.
“Spirochete antigens can be detected adjacent to ear cartilage in antibiotic treated Myd88-/- mice” (p. 3).
Translation: At the end of the study, the researchers took tissue samples from the ears of all the mice. They tested these samples for B. burgdorferi using both immunofluorescence staining (looking for antibodies) and culture techniques. The mice who were “sham treated” (not given antibiotics) tested positive. The mice who were treated with Ceftriaxone had negative cultures, but some “spirochete antigens” were detected in a deep layer of skin next to the ear cartilage. By “spirochete antigens,” they mean not live spirochetes but proteins (building blocks) left over from the bacteria that can cause the immune system to react. These antigens were found at a deeper level than where the imaging had earlier been peformed (which explains why they weren’t detected using imaging). The same antigens were detected in ear tissue from mice treated with Doxycycline. The researchers conclude that because the antigens were detected, but the bacteria could not be cultured (grown), it means whatever these spirochete remains were, they were incapable of multiplying because they had been too damaged by the antibiotics.
“Live imaging reveals antigen deposits but not motile spirochetes adjacent to cartilage of Myd88-/- mice after Doxycycline treatment for B. burgdorferi infection” (p. 3).
Translation: A separate experiment was conducted in which researchers studied mice between 2 and 10 weeks after finishing a 1 month course of Doxycycline. They used a technique called xenodiagnosis, where they let clean ticks bite the mice. They could find some B. burgdorferi DNA in the ticks that fed on the mice treated with Doxycycline, but when they studied the contents of the ticks’ guts, they could not find any spirochetes. By contrast, the ticks that fed on mice not treated with antibiotics had the bacteria in their bellies. In addition to using xenodiagnosis, they did the immunofluorescence test and cultures on this group of mice, and as before the cultures were negative and the immunofluorescence found antigens near the ear cartilage. This time, they used their imaging technique to look deeper under the skin, closer to the ear cartilage. In the sham treated mice, they found that there were motile (alive) spirochetes right next to the cartilage and large “deposits of nonmotile fluorescent material” where the skin meets the cartilage. In the antibiotic-treated mice, they saw no live spirochetes, but the same deposits were present next to the cartilage. So to see if these deposits would cause infection, the researchers transplanted skin from the infected mice (both those treated with Doxycycline and the sham-treated ones) into non-infected mice. Only the skin from sham-treated mice caused infection in the new mice. This shows that the antigens adjacent to ear cartilage in the mice treated with Doxycycline were not infectious.
“Spirochete antigens can be detected in joints of antibiotic-treated C3H Myd88-/- mice” (p. 4).
Translation: Here, the researchers decided to look at the knee joints of the mice to see if the same antigen deposits exist in antibiotic-treated mice. They looked at the knees of mice that had been infected for 4 months (which is a long time considering mice only live for about a year). When they treated these mice with Ceftriaxone, intravital microscopy showed that the spirochetes died off, but debris was left behind. They are pretty sure the spirochetes died off because the cultures were negative.
“Tissues from antibiotic-treated mice contain immunogenic and inflammatory B. burgdorferi antigens” (p. 4).
Translation: Finally, they wanted to test whether the deposits left behind in the knees of the mice actually contained B. burgdorferi antigens. They did this by immunizing new mice with knee tissue from the infected mice. They found that both the tissue from sham-treated and antibiotic-treated mice caused an IgG immune response to several B. burgdorferi proteins in the new mice.
Problems with the study:
1. It’s in mice. If you follow the research on B. burgdorferi, you’ll see that many of the studies are done in mice. That’s because it’s much less expensive to study disease in mice than in other animals. However, if we really want to learn about arthritis and B. burgdorferi in the human body, it would be better to do a study like this in Rhesus monkeys, which are much more similar to humans. Hopefully, this study will make it possible for some researchers to try to replicate this work in a primate model so that we can learn more.
2. The use of a “lab” strain of B. burgdorferi. They used a 297 strain of Borrelia burgdorferi which is “stable”–in other words, it’s not changing. It’s old and predictable. The problem is that most Borrelia in the wild are likely mutating and changing. They could even be developing resistance to antibiotics. After all, these organisms have been around for thousands of years; they are masters of adaptation. It would be much more interesting to do a study like this using a “wild” strain of B. burgdorferi, as this would more closely mimic the average patient’s experience.
3. The ambiguous blot analysis. The researchers used their own immunoblot to look at the antigens in the patellas (knees) of the mice. It’s not clear to me why they didn’t just use a Western Blot (since that’s the test used on us humans). Another odd thing they did was use a dilution of 1:1,000 for the blot, which is ten times the dilution used for other blots. Perhaps a lower dilution showed too many similarities between the sham group and the antibiotic group? In any case, for a study like this, I would expect more justification for these unusual choices.
4. The study’s “lack of heart” (and brain). I’m referring to the fact that the researchers failed to examine the effect of B. burgdorferi in the hearts of the mice. We know that, in addition to knee problems, patients with B. burgdorferi infections are at risk for a variety of heart problems, including myocarditis. The researchers were so bent on showing that antibiotics could kill the bacteria near joints, but what does that matter if the heart is still infected? If they truly believe that 24 hours of Ceftriaxone in mice eliminates B. burgdorferi, they missed a golden opportunity to show it by neglecting to examine the hearts–and the brains, for that matter. Not only did they ignore the heart, but they wasted valuable word space in their discussion section attacking the research design of Embers et al’s 2012 study of B. burgdorferi infection in Rhesus macaques. Now, the infamous monkey study is far from perfect, but they did do one thing right, which was to look at the heart tissue of the monkeys post-mortem–and guess what they found? In 3 out of 12 monkeys who were treated with antibiotics (that’s 25%), B. burgdorferi RNA could be detected in the heart. That’s despite the fact that in all 12 of the treated animals, C6 antibody titers decreased steadily over the course of treatment.
5. The use of septra/Sulfatrim. This one is a doozy. Earlier, I mentioned that the researchers added the antibiotic sulfamethoxazole-trimethoprim (Sulfatrim) to the mice’s drinking water “to reduce opportunistic infection”(p. 7). They claim that this drug has “no effect on B. burgdorferi infection or disease”(p. 7). I’m guessing they think that because of this 1996 study done in Austria. In that study, several species of Borrelia were evaluated to see whether they were susceptible to amoxicillin, azithromycin, cefotaxime, ceftriaxone, doxycycline, penicillin G sodium, roxithromycin, and trimethoprim-sulfamethoxazole (Sulfatrim) in vitro. The researchers used 30 different strains of Borrelia, but only 4 of those were Borrelia burgdorferi, and they were European B. burgdorferi at that. They found that B. burgdorferi was resistant to trimethoprim-sulfamethoxazole. Now, even though both ceftriaxone and trimethoprim-sulfamethoxazole were studied, the Austrian researchers didn’t investigate what would happen if you used both of these drugs on the Borrelia at the same time (which is what was done in the Yale study). In fact, after scouring PubMed, I was unable to find any synergistic Borrelia studies using ceftriaxone and trimethoprim-sulfamethoxazole. I did, however, find this study, also from Austria, published in 1997. They found that “trimethoprim was more active against Borrelia burgdorferi than against a sensitive strain of Escherichia coli, but sulfamethoxazole was not active against Borrelia burgdorferi.” In other words, one of the drugs that makes up Sulfatrim kills Borrelia, and the other doesn’t. The question is, if you add Ceftriaxone, does Sulfatrim start killing the Borrelia? To actually know whether or not Sulfatrim has an effect on B. burgdorferi when combined with Ceftriaxone, our Yale researchers would have had to do a synergy study, to see what would happen if you took the 50% kill rate of Doxycycline and Ceftriaxone, and add Sulfatrim. But they didn’t show that adding Sulfatrim didn’t affect the kill. So when they’re saying that “Ceftriaxone rapidly [within 24 hours] reduces pathogen burden in the skin,”(p. 5) they’re not taking into account that the Sulfatrim in the drinking water is also probably helping kill off spirochetes. It’s ironic how critical they are of other studies when their own study isn’t exactly “clean.”
6. The way Doxycycline was administered. Doxycycline was given to the mice in their drinking water. This means that the amount of Doxycycline in each mouse’s system depended on how much water it drank. The researchers said their reason for doing the Doxy in the water instead of force-feeding it to the mice twice a day was that when it was given twice daily, serum drug levels fell too low and they were not able to kill all the bacteria (p. 5). When I read this, I thought to myself, “Well, let’s see, how many humans do I know who are taking their Doxycycline through their drinking water? Oh, that’s right. None.” So here we have a study of Borrelia burgdorferi infection in mice in which the researchers choose not to give oral antibiotics because they believe not that the drug doesn’t work, but that the drug delivery system doesn’t work because it can’t get a high enough level of the drug into the blood stream. Yet, these are the same doctors who are saying that one month of oral Doxycycline should be enough to treat the same infection in humans. Curious, isn’t it? They even admit that one mouse may have stayed sick “due to a drinking pattern that led to inconsistent Doxycycline levels” (p. 5). So I guess either that mouse just wasn’t as thirsty as all the other mice, or he was eating his food and drinking his water in one sitting, and the food interfered with the drug absorption (as it can in people!).
7. They didn’t treat the arthritis. Okay, I get that the researchers were having lots of fun with their innovative real-time imaging technique. They tried to accomplish a lot with this study, and it already appears that they may have spread themselves a little thin. However, it bothers me that they spent no time examining ways to treat the arthritis caused by the deposits left behind by B. burgdorferi. In the world outside the laboratory, it doesn’t so much matter to people whether their arthritis is caused by live spirochetes or dead ones. They want to know what’s going to make them feel better. The study’s authors suggest that more antibiotics likely won’t work, but they don’t explore any alternatives, like steroids, for treating Lyme arthritis.
Some interesting (and some unexpected) implications:
1. Cool pictures. Intravital microscopy, the real-time imaging technology used in this study, is pretty nifty, and could be used in better-designed studies to find out a lot of useful information. The researchers in this study could even see some of the spirochetes changing into spherical forms, but they didn’t really investigate or discuss this in detail, beyond saying they don’t think those forms are bacterial cysts. It might be useful to have an entire study dedicated to investigating that.
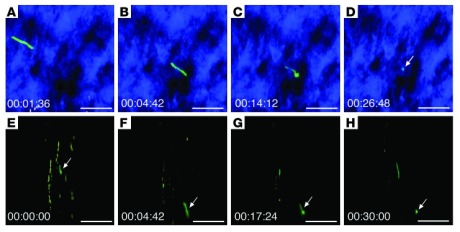
Image sequences of Bb914 (Borrelia burgdorferi) changing from an elongated spirochete to a spherical form. (Image via ncbi.nlm.nih.gov; J Clin Invest. 2012 July 2; 122(7): 2652–2660.)
2. Rethinking oral Doxycycline. It’s been my belief for a while now that oral antibiotics are just not as effective at killing Borrelia as IV antibiotics like Ceftriaxone. I’m not expecting everyone to agree with me on this, but let me tell you why I think so. While Doxycycline is by far the best choice among oral antibiotics for killing Borrelia (as it’s better at crossing the blood-brain barrier than many other drugs), there is an inherent disadvantage to all oral drugs because they have to be delivered through our digestive system. As a patient who took Doxy for a month, I can tell you that no matter how responsible and organized you are, it is very difficult to eat meals at the same time every day, to space the doses 12 hours apart, and to avoid ingesting things like milk that interfere with drug absorption. Reading Bockenstedt et al’s article made me further question the effectiveness of oral Doxy, as the researchers decided that oral Doxy twice per day would not be enough to keep serum drug levels consistent. Instead, they opted to deliver the drug through the mice’s water supply, which poses other problems with consistency. In any case, if it’s not good enough for lab mice, I don’t see how it’s good enough for humans.
3. A new drug combo? The study shows that in the presence of trimethoprim, you can rapidly kill Borrelia with Ceftriaxone. That means we should be doing more studies on how this works and whether it is safe for humans. There is always concern with killing off bacteria too rapidly because macrophages need time to clear the debris (which we think causes arthritis). However, this drug combination seems worth examining in other laboratory studies.
4. Location, location, location. The study doesn’t show that cartilage can be infected with Borrelia burgdorferi, but it does show that deposits are left over near cartilage after the bacteria have been disassembled by antibiotics. If the infection is in cartilage, that’s bad news, because there is no blood flow to cartilage, so it’s very difficult to eradicate an infection there. We need more studies that examine how this bacterium acts around cartilage.
5. Chronic Lyme? Contrary to what Yale alum and journalist Carole Bass would have you believe (Thanks to Becki from Bloody Lymey for opening my eyes to that one.), this study neither proves nor disproves the existence of Chronic Lyme disease, so despite the agenda that may underlie this study, patients need not see it as a threat. The study authors themselves admit in their Discussion section that they’re not quite sure what all their data mean: “The significance of B. burgdorferi DNA in xenodiagnostic ticks and in mouse tissues after antibiotic therapy is unclear” (p. 5). One possibility is that “Some B. burgdorferi DNA could remain intact if it is sequestered in cellular debris such as the GFP deposits.” They’re saying they think that the B. burgdorferi DNA they detected is just remnants of dead spirochetes that were preserved because they were stuck in the debris left behind by the antibiotics. However, they also admit to another possibility: “Alternatively, spirochete DNA could represent a minor subpopulation of B. burgdorferi that is not killed by the antibiotic treatment.” It’s a one-sentence admission in a 9-page paper, but it’s there–and it means that despite what these researchers think is going on, they still can’t say with 100% certainty that the antibiotics completely eradicated the infection.
What the study does show is that there are deposits in mouse tissue that the researchers insinuate are dead organisms (they have Borrelia antigens, are immunogenic, and don’t appear to be infectious). Because they don’t examine all of the tissue–including the cartilage, the heart, and the brain–it’s difficult to say whether they have completely eliminated the bacteria with antibiotics. What Embers et al showed in their primate model is that there seems to be persistence of spirochetes following 4 weeks of IV Ceftriaxone treatment and 8 weeks of Doxycycline. Until somebody does another study in Rhesus monkeys and proves that they’re wrong, that study stands.
6. Treating the arthritis. We know that reactive arthritis caused by Borrelia infections is a real phenomenon, and this study suggests that the cause is the debris left behind by spirochetes following antibiotic treatment. However, what patients and doctors alike need is access to information about how best to treat this unique form of arthritis. I’ve heard anecdotes from patients and doctors about the helpfulness of steroids like prednisone during or following antibiotic treatment, but there really isn’t enough research being done on this. It would be nice if researchers on both sides of the Chronic Lyme debate would pool their resources for the sake of better patient care.
I hope you enjoyed this installment of Tick-Lit Tuesday. It’s good to be back.
What has been your experience with Lyme or Tick-borne Relapsing Fever and reactive arthritis? What questions would you like to see addressed in future research?
Related articles
Choline Breakfast in Mom’s Kitchen 07/29/2012
Posted by thetickthatbitme in Choline Diet, Whole Person.Tags: Borrelia, Central Coast, choline, eggs, Food, health, Lyme, Sourdough, tomatoes
2 comments
I’m excited to be visiting my parents this week, especially because their kitchen is always filled with fresh fruits and vegetables. I missed the Central Coast’s superior tomatoes!
Choline count: 2 eggs (250 mg) + 1 tomato (12 mg) + sourdough bread (15 mg) = 277 mg of choline
Happy Sunday everybody!
Related articles
- This week’s choline diet highlights (thetickthatbitme.com)
- The Choline Diet: Herbivore Style (thetickthatbitme.com)
- You are What Your Mother Eats: Higher Choline Means Lower Cortisol For Baby (valerieberkowitz.wordpress.com)
06/10/2012
Posted by thetickthatbitme in Choline Diet, Reblogs.Tags: Borrelia, choline, diet, Lyme, medicine, nutrition, pregnancy, tick-borne
add a comment
Great post about the importance of choline intake during pregnacy.
Related articles
- Eat Your Eggs, Benedict! (thetickthatbitme.com)
- Omelet you in on these yummy high-choline recipes (thetickthatbitme.com)
- Snacking in the name of choline (thetickthatbitme.com)
Well, Babs, you’re trickier than I thought 05/01/2012
Posted by thetickthatbitme in Diagnosis, Peer-Reviewed, TBI Facts, Tick-Lit.Tags: Babesia, Blood donation, Blood transfusion, health, IFA, labs, Lyme, medicine, PCR, smear, tick
1 comment so far
Welcome to the second installment of Tick-Lit Tuesday, where I comb through PubMed so you don’t have to. Today’s topic: Babesia and Blood Transfusions. Now, I know I posted about Babesia in the blood supply just a few days ago, but an interesting study has since come to my attention (thanks, Dr. W), and the implications are a bit scary. Okay, get your popcorn and let’s begin.
The Issue:
It has been well-documented that the tick-borne protozoan parasite Babesia can be contracted through blood transfusions. Blood centers aren’t required to test donated blood for Babesia, but this may change in the future, as Babesia infections contracted through transfusions are on the rise. So if we were to test all donors for Babesia prior to donation, which tests should we rely on to detect this pesky parasite? Let’s look at the candidates.
IFA: IFA is an abbreviation for indirect fluorescent antibody test. This type of test can also be referred to as serologic (as in blood serum) testing. If you’ve had one of these tests for Babesia, it’s probably titled something like “WA1 IGG ANTIBODY IFA” (for B. duncani) or “BABESIA MICROTI ABS IGG/IGM” on your lab results. If you’ve had Babesia in the past and been treated for it, your antibody test might still read positive because your body is still making antibodies to the parasite. This is one of the reasons why most insurance companies refuse to pay for treatment for Babesia if your only positive test is the IFA. They think maybe you had a past infection that you got over, so you don’t need treatment. (The other reason they refuse to pay is that they’re jerks, to put it nicely.) I’ll talk more about why this is such a problem later in this post.

A stained blood smear on which B. microti parasites are visible in red blood cells. (CDC Photo: DPDx). Via CDC.gov.
Smear: When we talk about a smear for Babesia, we mean a Giemsa-stained thin blood smear. This test involves looking at blood samples under a microscope to see if there are any parasites hanging around. The problem with this test is that Babesia can infect fewer than 1% of your circulating red blood cells, so it could take many, many smears before any Babesia show up under the microscope. For more information about that phenomenon, read this.
PCR: This stands for polymerase chain reaction. It’s basically a DNA test that tries to identify whether a gene associated with Babesia is present in the blood. PCR has been found to be “as sensitive and specific” as blood smears for Babesia (see this study), which is not saying much, considering the tendency of Babesia to go undetected with smears.
Hmmm, for whom shall I cast my ballot, the antibody test insurance companies don’t trust, the inaccurate smear, or the inaccurate PCR? Choices, choices…
Today’s question:
Can the donated blood of someone with a negative PCR and negative blood smear still be infected with Babesia and cause Babesia infection in transfusion recipients?
(Hint: This is a leading question.)
Let’s talk about a study published in the journal Transfusion in December of 2011 called “The third described case of transfusion-transmitted Babesia duncani.”
Here’s what happened:
In May 2008, a 59 year-old California resident (I’ll call him Cal) with sickle-cell disease had some red blood cell transfusions. Cal’s only risk factor for Babesia was the transfusions; he didn’t have any tick exposure. In September of 2008, Cal was diagnosed with a Babesia duncani (WA-1) infection. The parasites were visible on a blood smear, the indirect fluorescent antibody (IFA) test was positive, and the PCR was positive for the Babesia gene. This launched a transfusion investigation in which doctors tracked down 34 of the 38 blood donors whose blood could have infected Cal with Babesia. One donor, a 67-year-old California resident (who I’ll call Don) had a B. duncani titer of 1:4096 (on the IFA test). What does a titer of 1:4096 mean? Well, if the antibody test for B. duncani is negative, the titer will be < 1:256. That means that Don’s antibody test was positive.
What the article abstract doesn’t tell you, which the full article does, is that both Don’s PCR and blood smear were negative for Babesia. How did the researchers prove definitively that Don had Babesia in his blood? They injected the blood into Mongolian gerbils, and were later able to isolate the parasite from the gerbils. Conclusion: Even though Don showed no symptoms of Babesia and both his PCR and smear were negative, his donated blood caused Babesiosis in both Cal and the gerbils.
Here’s why the study’s findings are important:
1. Clearly, blood smears and PCRs are not good indicators of whether someone is infected with Babesia. Why insurance companies think these tests need to be positive before they’ll pay for treatment is a mystery to me. There are probably a lot of people out there who’ve had positive IFAs but negative smear and/or PCR who were then not treated for Babesia because either the doctor, the insurance company, or both said they didn’t have an infection.
2. As far as the blood donation goes, if we don’t start screening out donors with positive Babesia IFAs, we’re going to continue to contaminate the blood supply with Babesia. It should be as simple as that. Been bitten by a tick? No blood donation for you. Positive IFA? No blood donation for you.
DZWSQK6QYS9G
Related articles
Four (surprising) places ticks hang out 04/30/2012
Posted by thetickthatbitme in Media, TBI Facts.Tags: Borrelia, Grizzly Peak, health, Lyme, mice, Permethrin, prevention, tick, Tilden Regional Park, yard
4 comments
Most people think you have to be hiking around in the woods to pick up a tick. In reality, ticks are a lot closer than you think. Here are four (possibly surprising) places where ticks hang out:
1. In your un-mown lawn. Ticks like to hide in vegetation to keep from drying out. Vegetation includes tall grasses, so don’t get lazy on the lawn upkeep!
2. In piles of fallen leaves. Yes, leaves are fun to jump in, and yes, the crunchy sound they make when you walk over them is lovely, but you (or your pet) could also be picking up ticks from leaf litter, so rake ’em up!
3. Anywhere mice or other rodents live. This includes wood piles, rock walls, crawl spaces, ground covers, abandoned vehicles, garbage, bushes, and palm trees. Mice also like to eat fallen fruit, so if you have fruit trees, be sure to dispose of any fruit that falls. If you have mice or rats in your home, chances are you have ticks, too. Here’s a more detailed list of mouse hiding places and what you can do to keep them away from your yard and house.
4. On and underneath wooden picnic tables and benches. To me, this is the creepiest one, because I’ve been to countless kids’ birthdays and neighborhood get-togethers in the park, and the last thing on my mind was tick exposure. If you don’t believe me when I say the risk is real, here’s an article abstract for a study conducted by Kerry Padgett and Denise Bonilla from the California Department of Public Health.
They collected ticks (some of which tested positive for Borrelia) from various areas in Berkeley’s Tilden Regional Park and found as many on wood surfaces as in leaf litter. If you’re planning on a day in the park, I recommend long pants and repellent with Permethrin.
If you’re spending time outdoors, it’s a good idea to check yourself for ticks as soon as you come inside. The University of Rhode Island’s Tick Encounter Resource Center has a great multimedia tool, the Tick Bite Locator, which suggests common places to check for ticks. They also have images of a variety of disease-carrying ticks (although the soft-bodied ones are missing) at different life stages.
Got a dog and not sure how to check him/her for ticks? WordPress blog After Gadget has a detailed explanation of how to do a thorough tick-check.
Be careful out there, everyone!
Related articles
Pink Deer and TBID Prevention 04/23/2012
Posted by thetickthatbitme in Media.Tags: deer, Fairfax County, Lyme, pink, prevention, tick, tick-borne illness, Virginia, Washington Post
2 comments
We all know (I hope) that an ever-increasing deer population means an ever-increasing tick population. We may not be able to stop the deer from multiplying (although I hear some are trying with bowhunting), but can we stop the ticks?

Fairfax Wildlife Biologist Vicky Monroe displays the day-glo pink pesticide that will show up on deer and any other animal who visits the county’s new feeders. Image via The Washington Post.
A March 26 article in the Washington Post describes a study the Fairfax County (Virginia) Wildlife Biologist’s Office (in collaboration with the county’s Disease Carrying Insects Program) is undertaking in which deer are attracted to feeders with corn and simultaneously treated with permethrin, a tick-killing pesticide. The twist? The pesticide has been dyed pink to allow for easier tracking of the deer. Fairfax County residents can expect to see not only pink deer, but also squirrels, raccoons, birds, and any other fauna that stop by for a snack.
How will this aid research on and prevention of tick-borne illness? Washington Post’s Tom Jackman explains:
On a couple of days every other month for the next three years, the pink deer will be harvested (or “killed,” in non-wildlife biologist terms) and autopsied. Deer organs will be tested and the remaining ticks will be sent to a lab for detailed analysis
Thus, the pink deer study will help the Fairfax County Wildlife Biologist’s office determine how effective the feeder-application of the pesticide is in killing disease-carrying ticks on the deer.
The study is costing the Fairfax County Health Department $380,000. For those in the county who have been affected by tick-borne infectious diseases (TBIDs), I’m sure this is not too high a price.
Would you support programs like this in your community? What is your county doing to control the vector population and prevent TBIDs?
Eat Your Eggs, Benedict! 04/22/2012
Posted by thetickthatbitme in Choline Diet, Whole Person.Tags: Anaplasmosis, benedict, Borrelia burgdorferi, Borrelia hermsii, choline, diet, eggs, HBO, inflammation, Lyme, mushroom, recipe, salmon
5 comments
If you know my story, you know that when I was diagnosed with B. hermsii and Anaplasmosis, my doctor put me on a high-choline diet. Why choline, you ask? Choline is a B vitamin that aids in the transmission of nerve impulses from the brain through the central nervous system–this process is essential to functions like memory and muscle control. Since Borrelia like to attack the central nervous system, choline is especially important for people with (past and present) B. hermsii and B. burgdorferi infections. People who eat diets high in choline have also been shown to have lower levels of inflammation (like inflammation of the joints in Arthritis) than people who don’t. You can read more about choline here.
Enter the Benedict. It is by far my favorite egg-based dish, and I enjoy making it at home just as much as I do eating it for brunch in a fancy restaurant.
One large poached egg has 100 mg of choline, so if you eat two, you get about half of your recommended daily amount (425 mg for women, 550 mg for men). Add to that other high-choline foods like smoked salmon (129 mg), Canadian bacon (39 mg), portabella mushrooms (39 mg), spinach (35 mg), asparagus (23 mg), avocado (21 mg), and tomato (6 mg) to get your choline fix!
Here are my top five Benedicts:
1. Old Fashioned but Fried
for those mornings (or afternoons, or evenings!) when I’m feeling traditional, yet lazy
I learned this simple recipe from my mother, and it
brings back all kinds of fond childhood memories. A toasted whole-wheat English muffin, topped with pan-fried Canadian bacon and over-easy eggs (make sure they’re still a little runny, because that’s the best part). The hollandaise sauce I usually make with one of those sauce packets you can find in the grocery store (next to the gravy packets). It’s easy–you only need to add milk and butter–and, in my opinion, it tastes better than the from-scratch hollandaise recipes I’ve tried. Because of the butter and bacon, this is a slightly fattening meal, so I balance it with a side of boiled asparagus, which tastes delicious with the hollandaise sauce and adds 23 mg of choline to this meal!
Choline count: eggs 200 mg + Canadian bacon 39 mg + asparagus 23 mg = 262 mg of choline
2. Crab Benedict
for when I’m feeling crabby or rooting for the Terps
I’ve never made this one at home, but I’ve had it at Toasties Cafe, and it is delicious!
Choline count: eggs 200 mg
3. Portabello Mushroom Benedict
for the fungus-lovers amongus
If you’re looking for a meatless meal or just craving these yummy mushrooms, this is the Benedict for you. Check out Jackie Dodd’s recipe at TastyKitchen.com, which also includes spinach, tomatoes, and Sriracha for a kick!
Choline count: eggs 200 mg + portabello mushrooms 39 mg + spinach 35 mg = 274 mg of choline
4. Tomato Avocado Benedict
because I’m a California girl
My mouth was watering as I scrolled through SoupBelly.com’s deliciously illustrated recipe for this west-coast Benedict. If you want to make it even more California, use sourdough English muffins.
Choline count: eggs 200 mg + avocado 21 mg + tomato 6 mg = 227 mg of choline
5. Eggs Hemingway
for when I’m feeling literary
This one may seem a bit fishy, but I assure you it’s delicious and packed with choline. It’s also called Norwegian Benedict. Here’s a recipe at food.com that includes not only salmon but spinach, too!
Choline count: eggs 200 mg + smoked salmon 129 mg + spinach 35 mg = 364 mg of choline
Now that I’ve made myself really hungry, I’m going to go make my own Benedict. Hope you enjoy these eggcellent (sorry, I couldn’t resist) high-choline meals!
Curious about Tick-borne Infections? 04/21/2012
Posted by thetickthatbitme in TBI Facts.Tags: Borrelia burgdorferi, Borrelia hermsii, diagnosis, facts, infection, Lyme, Lyme Disease, TBI, tick-borne, treatment, ugly stepsister
2 comments
Happy Saturday, loyal readers!
I thought I’d point out that I’ve added a new section to the blog: Infection Fact Sheets. One of my goals with this blog is to give you, my readers, access to as much factual information about tick-borne infectious diseases–or TBIDs, as I like to abbreviate them–as possible.
Since you’ve stumbled upon this blog, I’m sure you’ve heard of Lyme Disease, but do you know the name of the bacterium that causes it? Are you familiar with the common and not-so-common symptoms? What about the different drugs that are used to treat this infection? Check out the fact sheet here.
And let’s not forget Borrelia hermsii, which I consider to be like Lyme’s neglected ugly stepsister. Nope, no press for Ms. B. hermsii… Take pity on her (or if not her, me, a hermsii survivor) and pay a visit to her fact sheet.
If I were truly going to put my teacher hat on and plan a lesson for you, I’d tell you to make a K-W-L chart and take notes!
Once you’re done with the Borrelia sisters, you’ll probably be hungering (or worrying?) for more TBID info. Here’s a list of what’s to come: Anaplasmosis, Babesiosis (WA-1), Ehrlichiosis, Rickettsia (Rocky Mountain Spotted Fever), and more!
This Season’s Ticking Bomb – WSJ.com 04/19/2012
Posted by thetickthatbitme in Media.Tags: Borrelia, CDC, hiking, infection, Lyme, pets, prevention, tick, tick-borne, Wall Street Journal
add a comment
Looking forward to spring? I’ve really been enjoying the extra daylight and walks with my dog, Lucy, after dinner, and it was so nice on Easter to be able to wear a dress without my legs getting cold!

Lucy is ready for a walk.
Nice weather, however, comes at a price. An article published last month in the Wall Street Journal explains how warming weather will contribute to an increase in tick population (and likely an increase in the number of tick-borne infections) this spring. You can (and should) read the full article here.
Here’s an interesting tidbit about a study the Centers for Disease Control are doing:
The CDC is conducting the first study of its kind to determine whether spraying the yard for ticks can not only kill pests, but also reduce human disease. Participating households agreed to be randomly assigned a single spray with a common pesticide, bifenthrin, or one that contained water, without knowing which they would receive.
Paul Mead, chief of epidemiology and surveillance activity at CDC’s bacterial-illness branch, says preliminary results from about 1,500 households indicate that a spray reduced the tick population by 60%.
“But there was far less of a reduction in tick encounters and illness,” indicating that even a sharp drop in tick populations leaves infected ones behind. “We may have to completely wipe out ticks to get an effect on human illness,” he says. The CDC is enrolling households for a second arm of the study and expects final results late in the fall. Organic repellents such as Alaska cedar are also being tested in other studies.
The article includes an interactive graphic with some suggestions for how to avoid tick bites in your backyard:
- Store firewood and bird feeders (birds carry ticks too!) away from the house.
- Keep leaves raked and grass mown.
- Restrict use of plants that may attract deer.
- Keep pets away from wood (and woods) and use tick repellant.
- Use decks, tile, and gravel close to the house.
- Seal up any holes in stone walls that mice might want to nest in. (And make sure your house is rodent-free!)
- Shower immediately after spending time outdoors in possibly tick-infested areas.
- Wash and dry clothing worn for hiking or golfing at high temperatures.
I’ve been trying a natural, non-toxic flea and tick repellant on Lucy (and myself) that’s made from cedar oil. What will you be doing this spring to avoid ticks (and thereby tick bites)?